Pre-configured for FDA, ISO, and GxP compliance

One Platform. With You Every Step of Your MedTech Journey.
Bringing a medical device to market is complex, but staying there is even more challenging. Fragmented tools, regulatory hurdles, and compliance risks slow down innovation, drain resources, and increase the likelihood of costly delays.
QuickVault unifies design, regulatory, and quality management into a single, seamless platform—eliminating inefficiencies and giving MedTech companies the structure needed to launch, scale, and achieve commercial success.
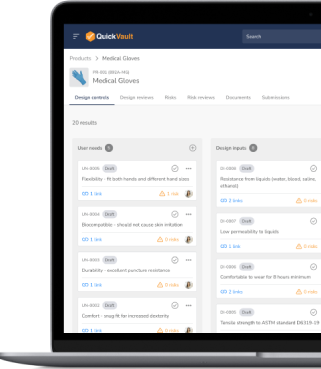
Design With Confidence. Build for Success.
Bringing an idea to life requires more than innovation—it demands structured compliance, seamless collaboration, and complete traceability. QuickVault ensures every design decision is documented and risk is controlled at all times.
Capture and manage all design elements in one place. QuickVault automatically generates your design files while maintaining version control, traceability, and regulatory alignment—eliminating compliance risks from day one.
Start Designing
Facilitate seamless collaboration with automated approvals and real-time feedback tracking. QuickVault keeps your team aligned and provides the guardrails to ensure all regulatory requirements are met before advancing.
Optimize Reviews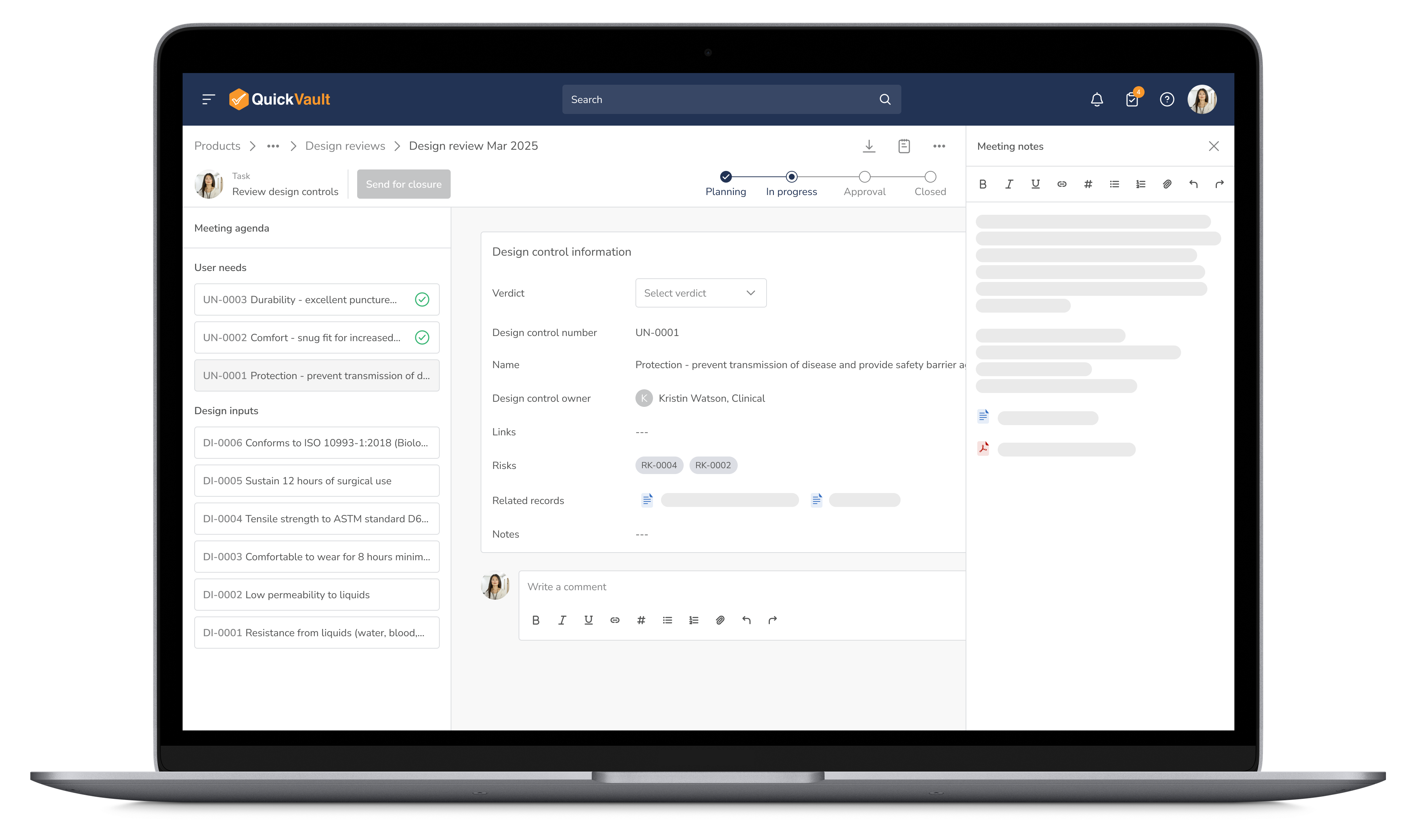
Identify and mitigate potential risks before they become setbacks. QuickVault’s integrated risk management workflow enhances product safety while streamlining compliance.
Manage Risks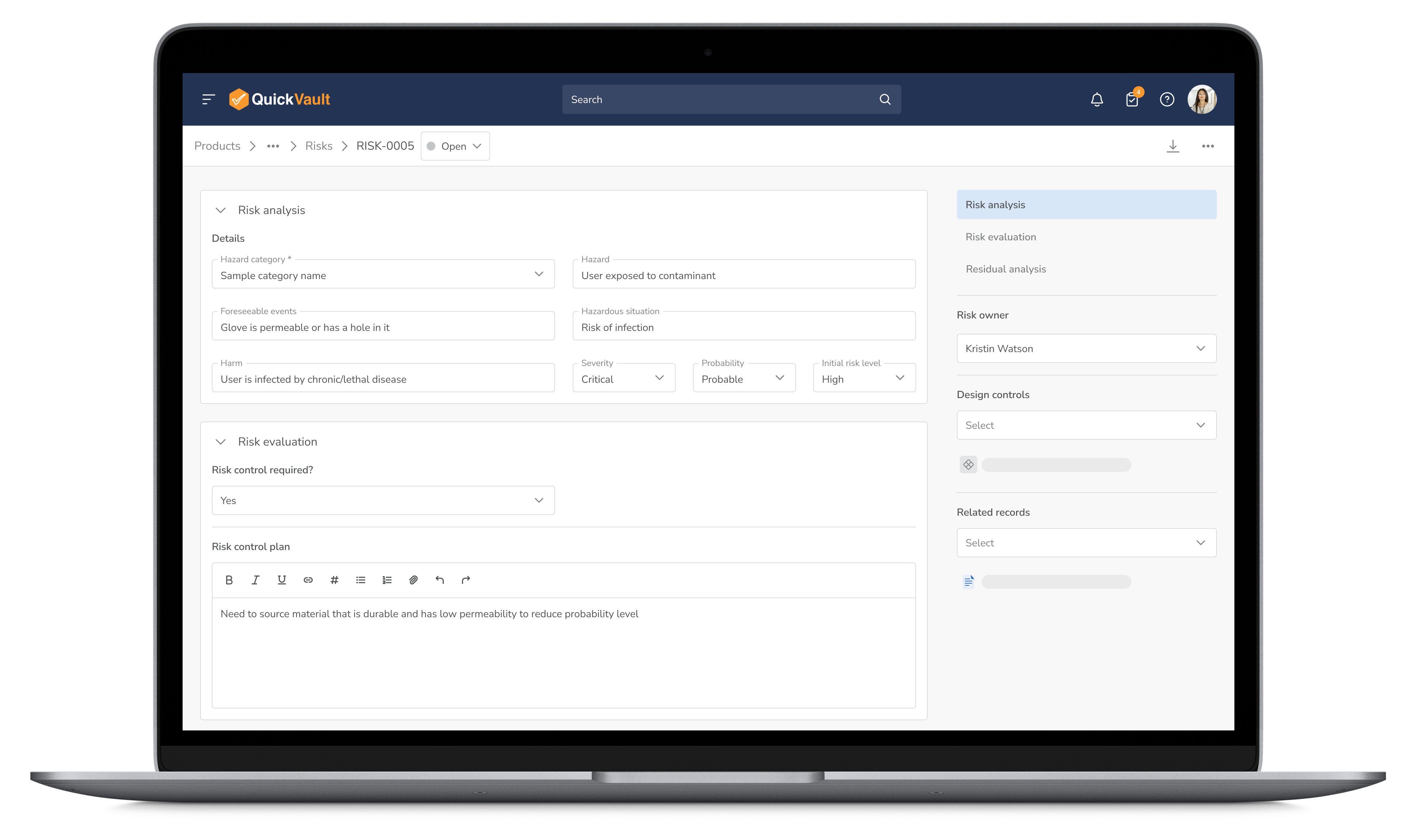
Designed for immediate use with continuous updates
Teams stay connected with structured workflows
Regulatory Submission Without the Complexity.
Regulatory approval is one of the biggest hurdles in MedTech. Disorganized documentation, submission delays, and compliance gaps can put your go-to-market strategy at risk. QuickVault streamlines the entire submission process, ensuring a structured, efficient path to approval.
Organize every submission document in a fully validated system with version control, audit trails, and real-time tracking. Never scramble for missing files again.
Manage Documents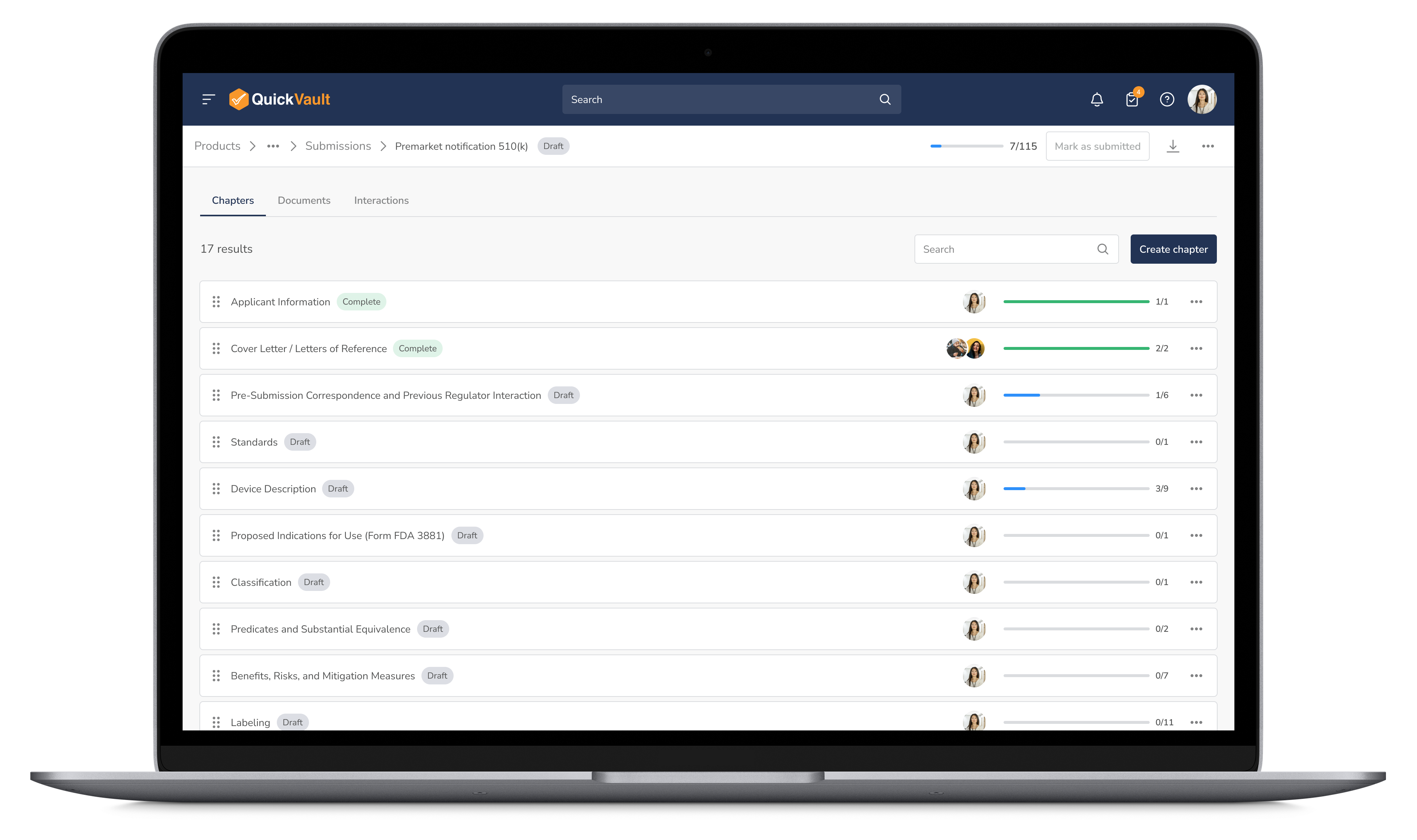
Respond to regulatory inquiries with confidence. QuickVault ensures all communications with the FDA and other regulatory authorities are organized and collaborative.
Stay Organized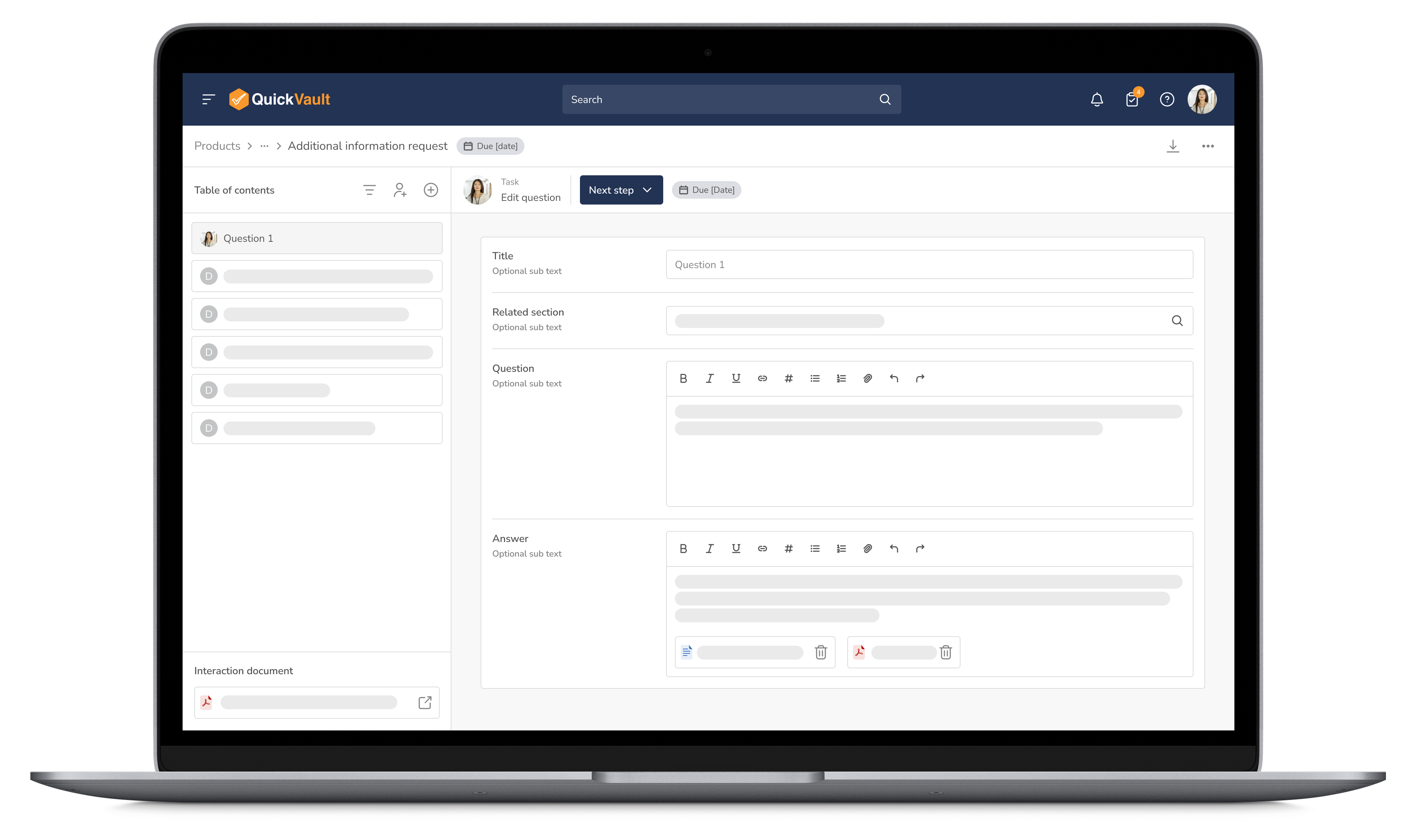
Simplify the product submission process with pre-configured workflows, automated tracking, and built-in compliance reporting to reduce approval timelines.
Streamline Submissions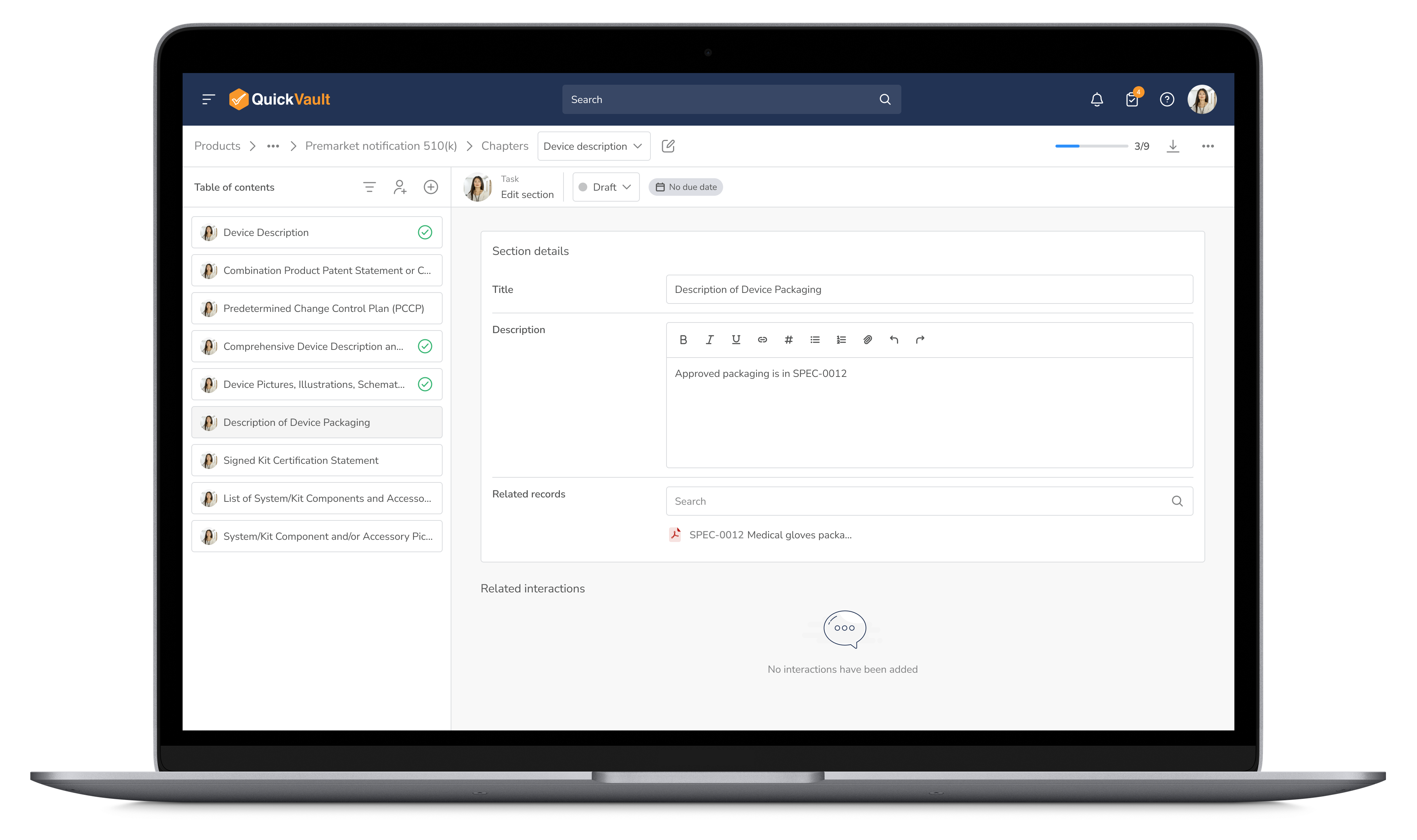
Keep documentation structured and submission-ready
Built-in FDA and ISO best practices ensure approval success
Automate submission tracking, approval workflows, and compliance reporting to speed up your time to market.
Quality and Operations That Scale With Confidence.
Getting to market is only half the battle—maintaining compliance, managing quality, and mitigating supply chain risks is essential for long-term commercial success. QuickVault ensures operational excellence and regulatory adherence at every stage of your post-market journey.
Ensure all production-related documentation is version-controlled, audit-ready, and easily accessible to maintain compliance and efficiency across operations.
Manage Documentation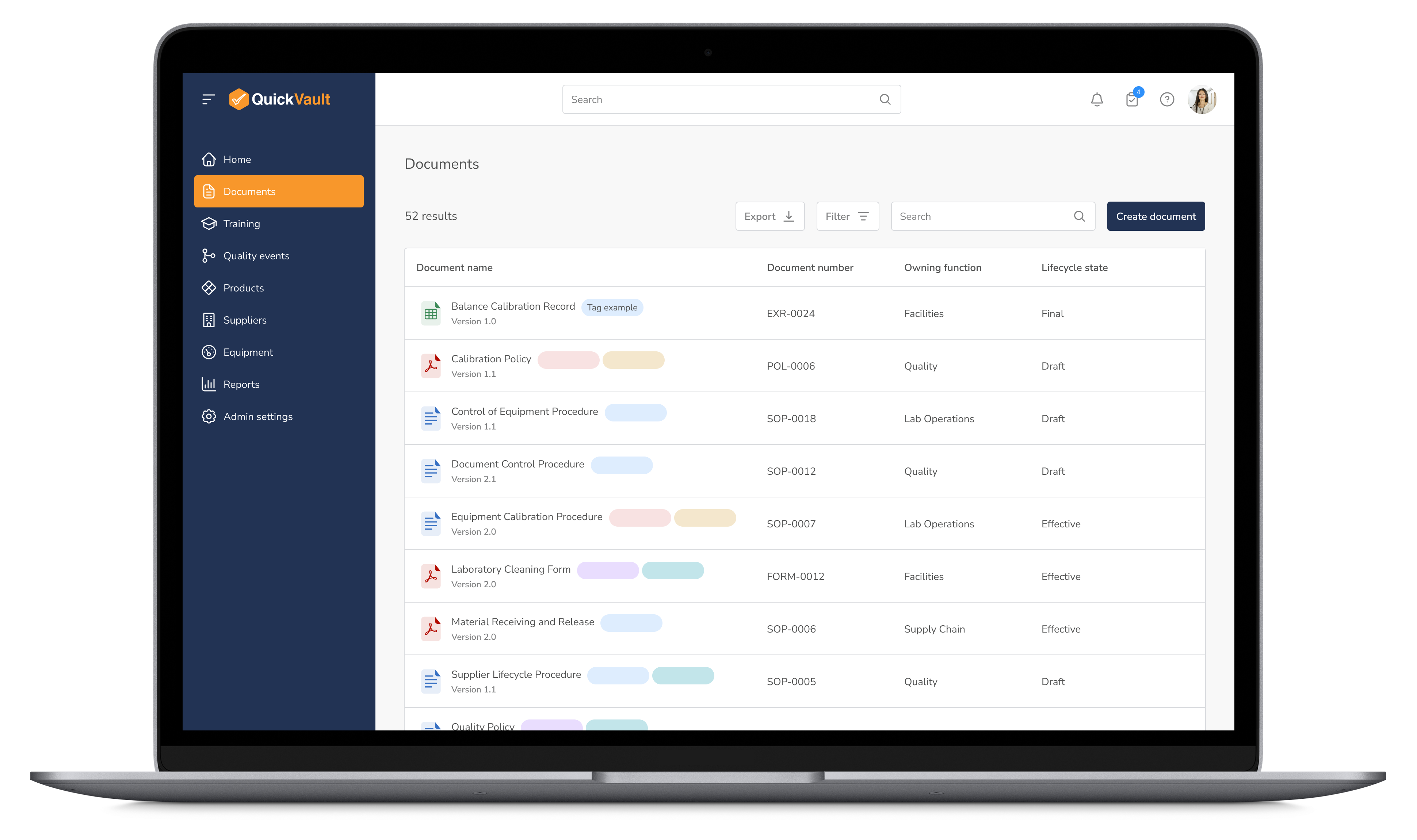
Equip your team with the knowledge and skills they need to maintain compliance. QuickVault automates training assignments, progress tracking, and certification management, ensuring every employee is up to date with the latest regulatory requirements.
Optimize Training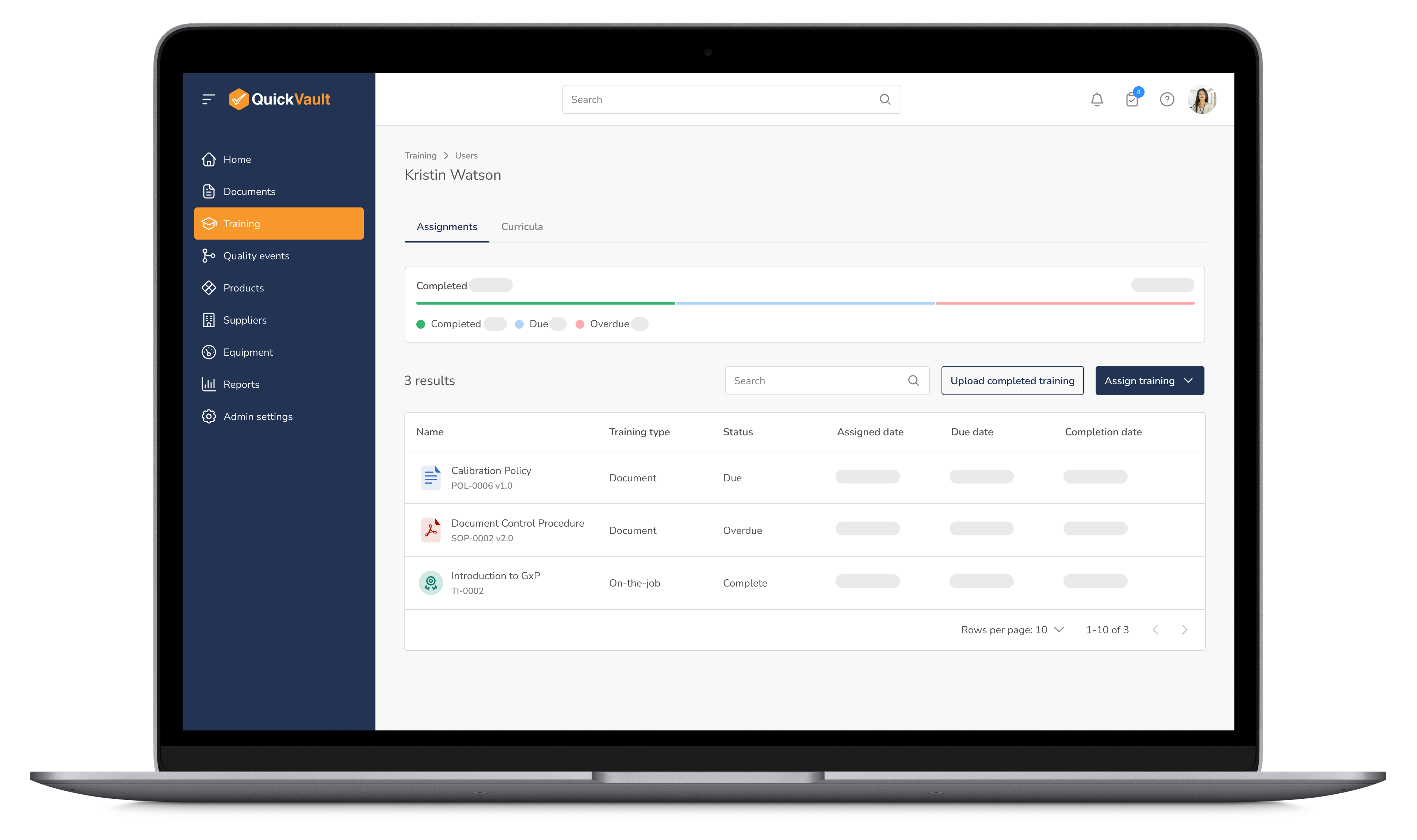
Proactively manage quality and mitigate compliance risks with structured workflows for CAPA, customer complaints, non-conformance management, and post-market surveillance. QuickVault provides an integrated approach to quality management, helping you ensure regulatory alignment at every stage of production.
Manage Quality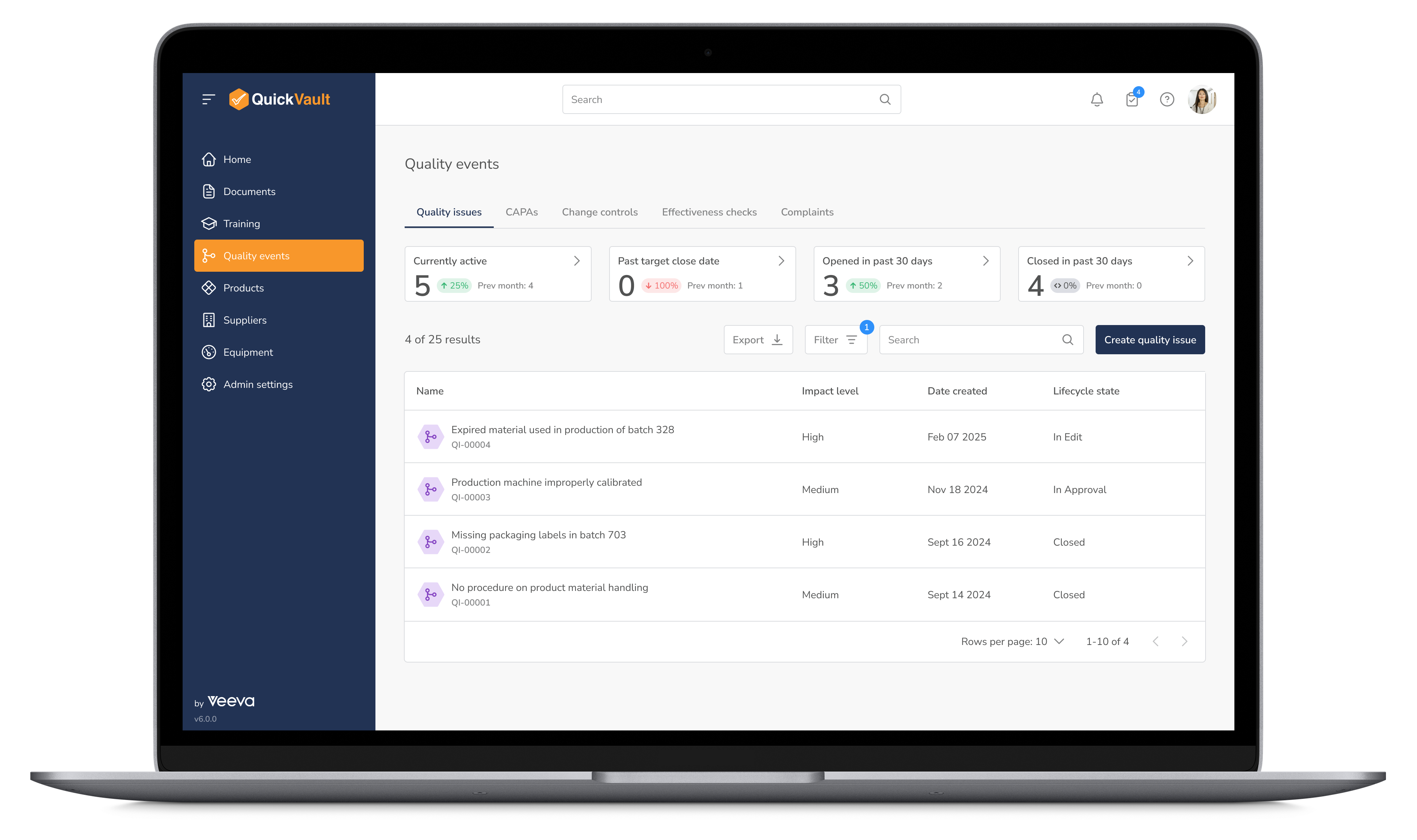
Monitor supplier performance, track equipment compliance, and manage audit schedules in real time. QuickVault helps mitigate supply chain risks and ensures that all production partners meet regulatory and quality requirements.
Manage Suppliers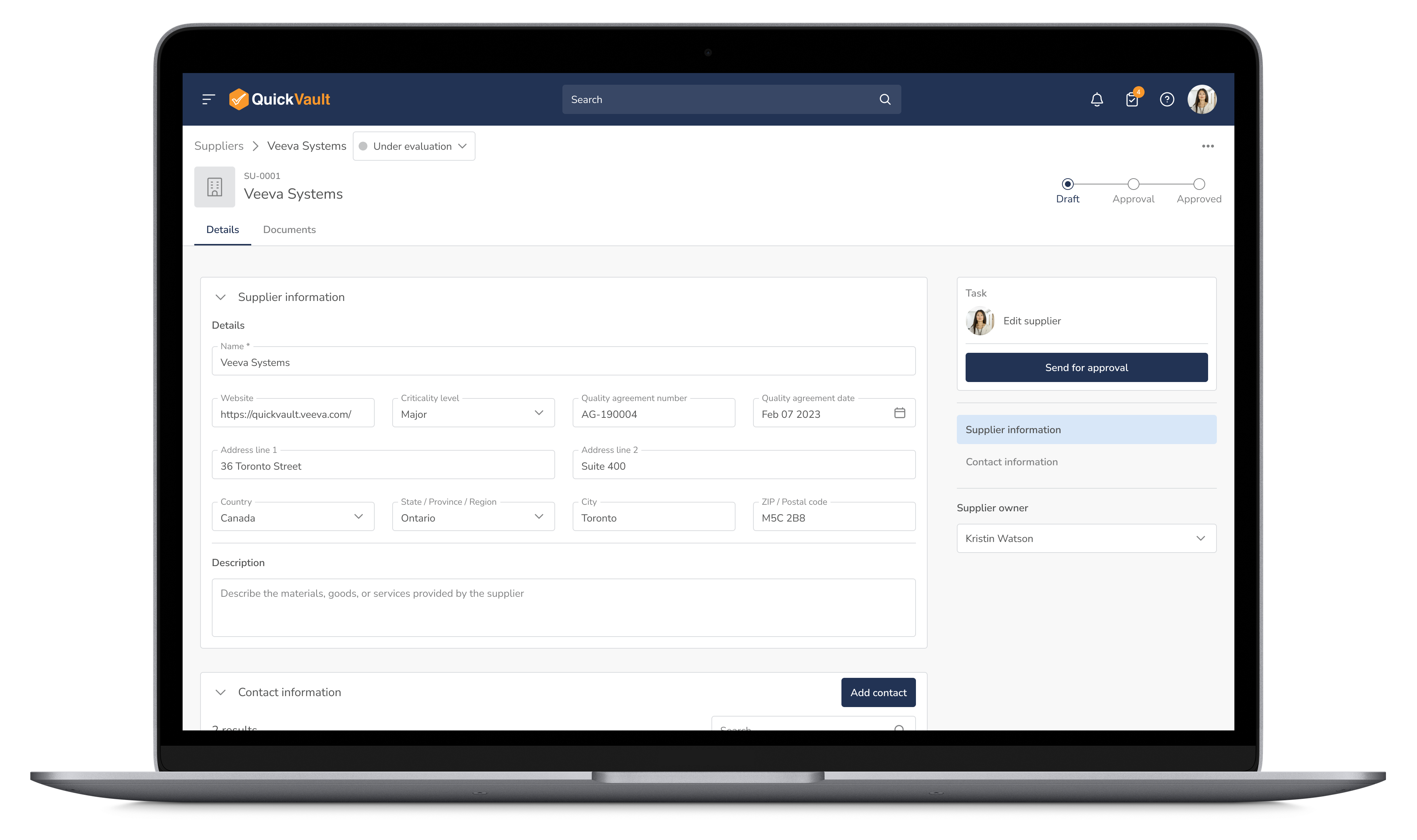
Ensure continuous adherence to FDA and ISO requirements
Centralize records, training certifications, and quality events
Identify and address quality issues before they escalate
Maintain operational excellence as production expands
QuickVault is a Trusted Authority in Medtech
Frequently Asked Questions
-
How does QuickVault support medical device companies?
QuickVault unifies design, regulatory, and production processes into a single platform, helping medical device teams streamline compliance, accelerate market entry, and scale operations efficiently. Every step is supported by built-in FDA, ISO, and GxP best practices, so you can move quickly without risking quality.
-
How does QuickVault help IVD companies manage ongoing compliance?
IVD companies face constant regulatory updates and growing expectations for traceability. QuickVault simplifies compliance by centralizing documentation, automating version control, and providing structured workflows for design, quality, and submissions. This helps IVD teams stay audit-ready while keeping development timelines on track.
-
What is QuickVault Hub for service providers?
QuickVault Hub is a shared collaboration environment that allows service providers to work directly with their MedTech clients on design, compliance, and regulatory projects—all within a validated, secure platform. This reduces inefficiencies, versioning issues, and compliance risks, enabling providers to deliver results faster and build stronger client relationships.
-
Is QuickVault suitable for startups with limited compliance experience?
Yes. QuickVault is purpose-built for MedTech startups and growing teams. The platform is pre-validated and ready to use from Day 1, with pre-built templates, guided workflows, and expert support to help teams achieve compliance—even without prior regulatory expertise.
-
Will QuickVault scale as my business grows?
Absolutely. QuickVault is designed to grow with you. Whether you’re launching your first device or expanding your product line globally, the platform adapts to support increasing complexity—without slowing you down.
The platform scales alongside your team, and our flexible subscription model ensures you always have the right level of support—without being locked into long-term contracts.
-
How does QuickVault reduce submission and approval delays?
QuickVault removes the guesswork from regulatory submissions by automating document management, ensuring version control, and providing submission tracking tools—so you can prepare faster, respond to inquiries quickly, and reduce the risk of costly delays.
-
Can QuickVault support different regulatory requirements across markets?
Yes. QuickVault is built to meet FDA, ISO, and GxP standards while also offering configurable workflows to support regional variations. Whether you’re preparing a 510(k), CE Mark submission, or aligning with MDR/IVDR, QuickVault ensures your quality and regulatory processes stay compliant.
One Platform for MedTech Success.
From initial concept to full-scale production, QuickVault eliminates compliance complexity and ensures a seamless path to market and beyond.
With a single, validated solution for design, regulatory, and quality management, your MedTech company can innovate faster, reduce regulatory risk, and build for long-term success.