MedTech Solutions
Built for Speed & Compliance.
Proven for MedTech Success.
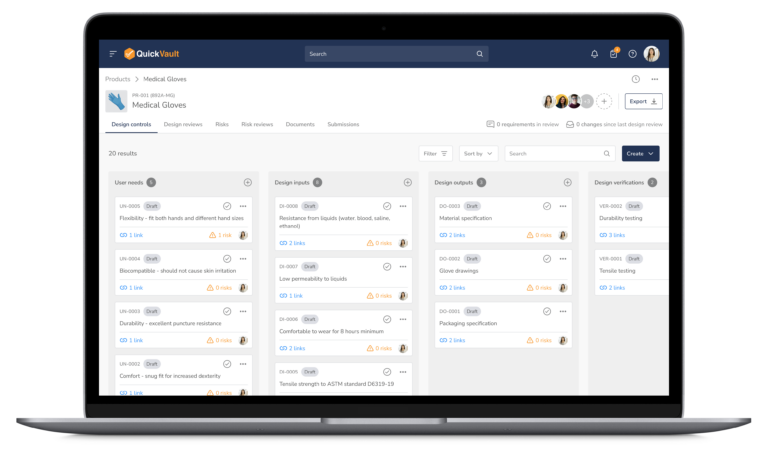
Bringing a medical device to market can be complex, involving strict regulations, detailed documentation, and careful risk management. QuickVault by Veeva simplifies the process, offering catered solutions built to accelerate MedTech success.